|
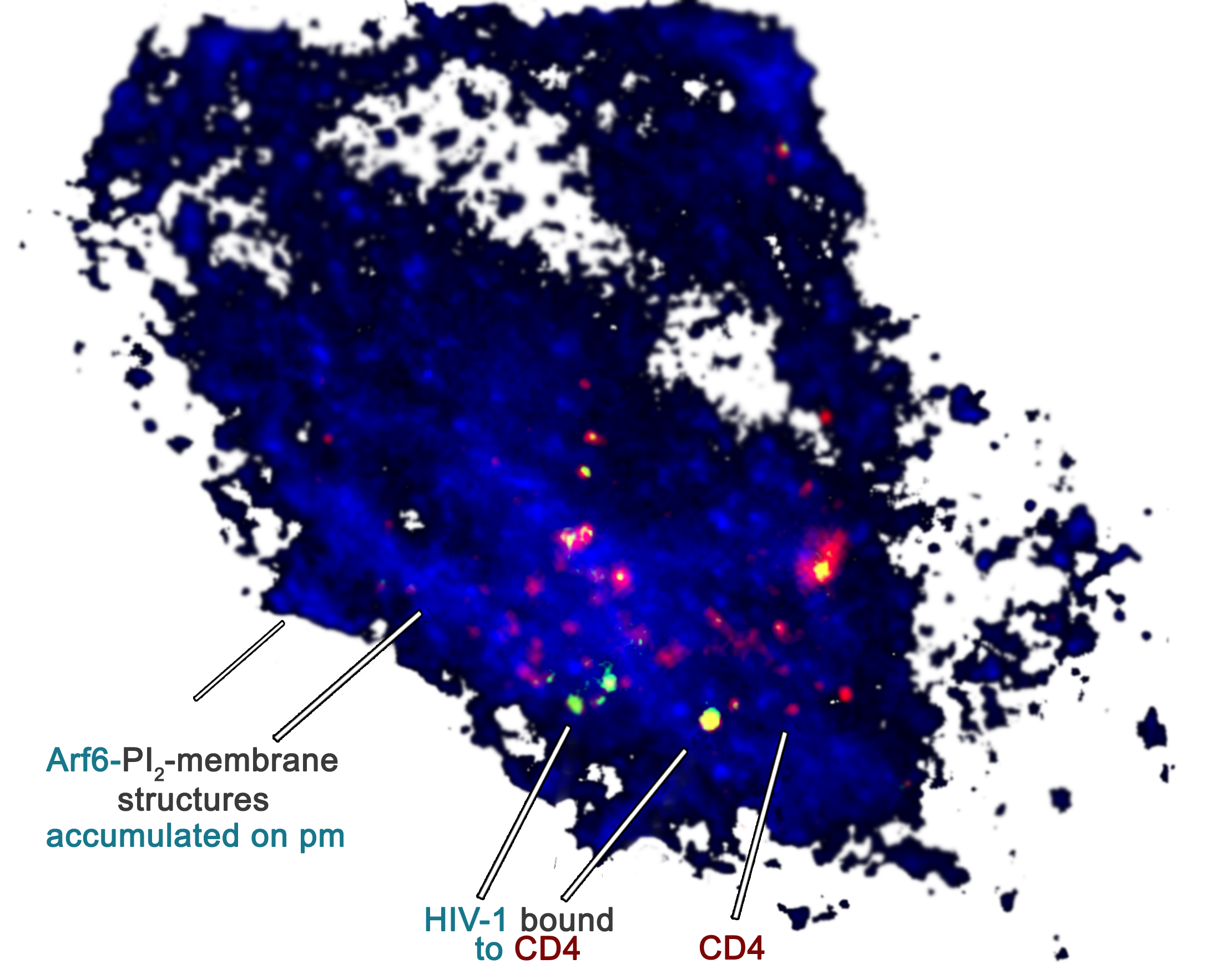
HIV-1 requires Arf6-mediated membrane dynamics to efficiently enter and infect T Lymphocytes. MBoC Laura García-Expósito et al. 2011. Image. PIP2-associated plasma membrane (pm) structures accumulate in cells over-expressing the Arf6-T44N mutant, rendering them refractory to CD4-dependent HIV-1 fusion and entry without affecting cell-surface CD4/virus interaction. TIRFM image shows the inhibition of the CD4-dependent productive HIV-1 uptake due to perturbed Arf6-coordinated PIP2-membrane trafficking, by using fluorescent HIV-1-Gag-EGFP viral particles in permissive TZMbl (CD4+/CXCR4+/CCR5+) cells, expressing the fluorescent CD4-DsRed molecule together with the Arf6-T44N mutant and the PH-ECFP probe, which allows to monitor PIP2/Arf6-membrane structures (merged image from Figure 8D, rotated 90º clockwise).
The ICV Group
|
Welcome to the homepage Cellular and Viral Immunology Lab
- To study cellular events that regulate HIV-1 fusion, entry and infection processes, by determining the functional role of cytoskeleton reorganization (and associated cellular factors: adaptors, kinases, phospahatases, GTPases, etc) and plasma membrane dynamics (i.e.; vesicle formation and trafficking). Generally our research focuses on the characterization of the dynamic mechanism of the viral-cell membrane fusion process, during early HIV-1 infection, and to determine the necessity of plasma membrane dynamics and the existence of a cortical structure to generate the appropriate membrane tension/fluidity in target cells to promote effecient pore fusion formation, entry and infection. - To determine and characterize new potential and natural retriction factors with anti-replicative HIV-1 activity or that selectively desestabilize viral production. - To determine the events underlying signal transduction through CD4, CXCR4 and CCR5 viral receptors during early HIV-1 infection; and their functional involvement in HIV-1 replication and spread, and in cell migration by their own natural ligands (during inflammation and metastasis biological processes). - To study the regulatory mechanism of the anti-HIV-1 activity of chemokines, such as SDF-1, MIP-1 and RANTES, natural ligands of CXCR4 and CCR5, mediated by different cellular factors (heparan sulphate and leukocyte proteinases). This research focuses on the “Logical Design of new antiviral molecules” based on the antiviral mechanisms exerted by these chemokines, suh as SDF-1 (CXCL12); e.g.: a study of the mechanism of occupation of the HIV-1 CXCR4 coreceptor by its natural ligand CXCL12, and the envelope of HIV-1 (protein gp120), which could help to develop new anti-HIV-1 drugs or alternative anti-AIDS strategies. Low-scale screenig of new natural or derivatives by semi-synthesis anti-HIV-1 molecules that exert their anti-HIV-1 activity by blocking early viral fusion and entry events of the HIV-1 life cycle. - Key words: anti-HIV-1 natural restriction factors; inhibition of viral infection, replication and spreading; viral auxiliary proteins. |
|
|
|